The Athero-Resilience Project: Systems Approaches for the Discovery of Atheroprotective Targets
Resilience to Atherosclerosis
Atherosclerosis (ASS) is the leading cause of myocardial infarction, stroke, and premature death. While the molecular and cellular mechanisms of ASS are incompletely understood, research has successfully focused on lipids, blood pressure, diabetes, and inflammation as major causes of vascular injury and plaque formation. Indeed, lowering blood pressure, reducing low-density lipoprotein cholesterol (LDL-C), smoking cessation, the use of novel anti-diabetic drugs, and even anti-inflammatory approaches have been shown to reduce major adverse cardiovascular events (MACE). However, in spite of all this, there is a substantial remaining cardiovascular (CV) risk with up to 20% of patients experiencing myocardial infarction, stroke, or death in the first year after an acute coronary syndrome (ACS). Notably, although CV risk factors are closely related to ASCVD and mortality, there is a significant variation in individual data. Even in familial hypercholesterolemia, some never develop an ACS or die of ASCVD. This obvious epidemiological observation has hardly been considered, as research has focused only on vascular injury as the sole cause of ASCVD.
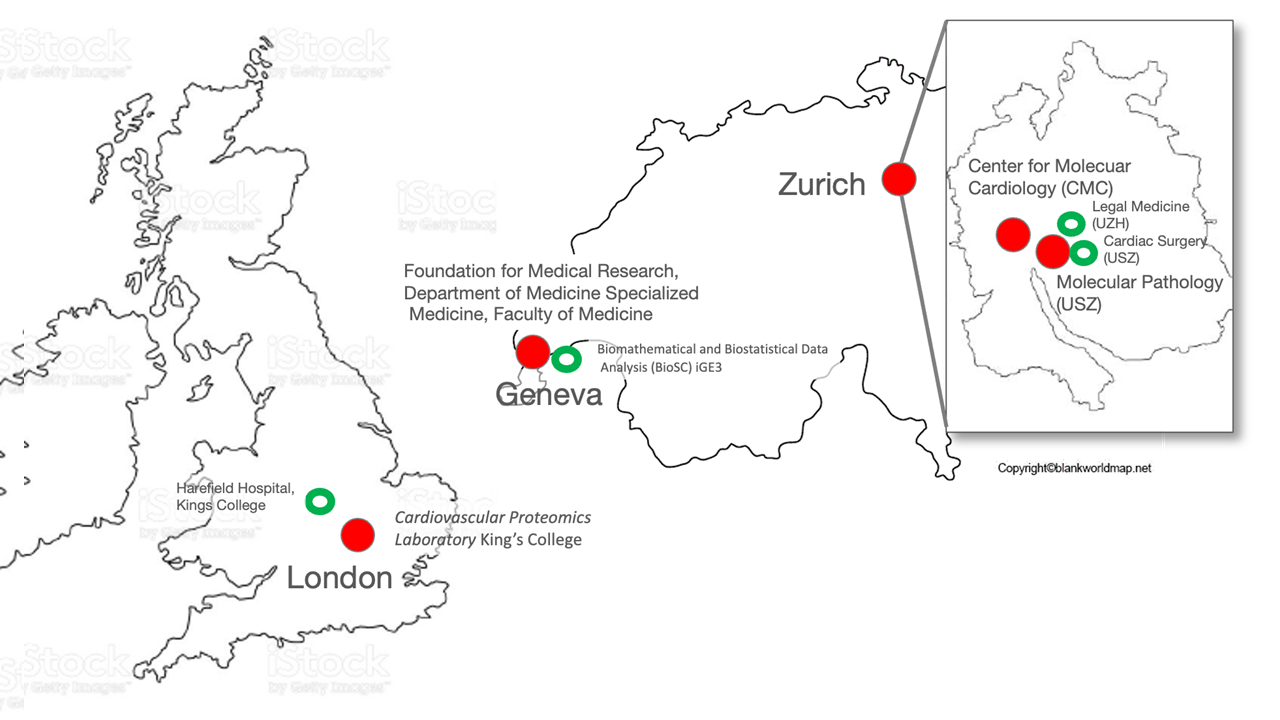
With this project, we aim to decipher the protective factors of the IMA providing resilience to ASS using a multiomics approach. We have sequenced the RNA profiles of the IMA, coronary and radial arteries, and saphenous veins, and aim to understand, using bioinformatics, the differentially expressed and activated pathways of these blood vessels that are prone or not to ASS. We will now confirm these results at the protein level using proteomics and subsequently verify the biological activity of candidate proteins in endothelial and vascular smooth muscle cells derived from these arteries in culture. To that end, we brought together three Centers with complementary expertise. In Zurich, the Center for Molecular Cardiology has a long-standing experience in the vascular biology of the IMA, coronary artery, and saphenous vein, collaborating with CV Surgery, Molecular Pathology, and the Functional Genomics Centre. Geneva has specific expertise in single-cell multiomics of endothelial and vascular smooth muscle cells at different anatomical locations. Finally, the King’s College proteomic service in London, UK, will perform extensive proteomic studies of these tissues.
The extensive complementary and interdisciplinary expertise of the involved Centers will provide a knowledge platform, addressing the question of what protective factors are expressed in the IMA but not in other arteries prone to ASCVD, as a basis for novel therapeutic strategies in ASS to further reduce the remaining CV risk.
Participating Institutions
- Center for Molecular Cardiology, University of Zurich
- University Hospital Zurich, University Heart Center
- University Hospital Zurich, Institute of Pathology and Molecular Pathology
- University of Geneva, Division of Cardiology - Foundation for Medical Research
- King's College London, Cardiovascular Proteomics
- Harvard Medical School, Channing Division of Network Medicine
Possible Mechanisms of Athero-Resilience

Fueled by the global surge in aging, atherosclerotic cardiovascular disease reached pandemic dimensions, putting affected individuals at enhanced risk of myocardial infarction, stroke, and premature death. Atherosclerosis is a systemic disease driven by a broad spectrum of factors, including cholesterol, pressure, and disturbed flow. Although all arterial beds encounter a similar atherogenic milieu, the development of atheromatous lesions occurs discontinuously across the vascular system. Indeed, the internal mammary artery possesses unique biological properties that confer protection to intimal growth and atherosclerotic plaque formation, thus making it a conduit of choice for coronary artery bypass grafting. Its endothelium abundantly expresses nitric oxide synthase and shows accentuated nitric oxide release, while its vascular smooth muscle cells exhibit reduced tissue factor expression, high tPA (tissue-type plasminogen activator) production, and blunted migration and proliferation, which may collectively mitigate intimal thickening and ultimately the evolution of atheromatous plaques. We aim here to provide insights into the anatomy, physiology, cellular, and molecular aspects of the internal mammary artery, thereby elucidating its remarkable resistance to atherogenesis. We propose a shift in perspective from risk to resilience to elucidate the mechanisms of atheroresistance and ultimately identify novel therapeutic targets that are currently not addressed by existing remedies.